EQBMED Innovations
The EQBMED Site Maturity Assessment Model
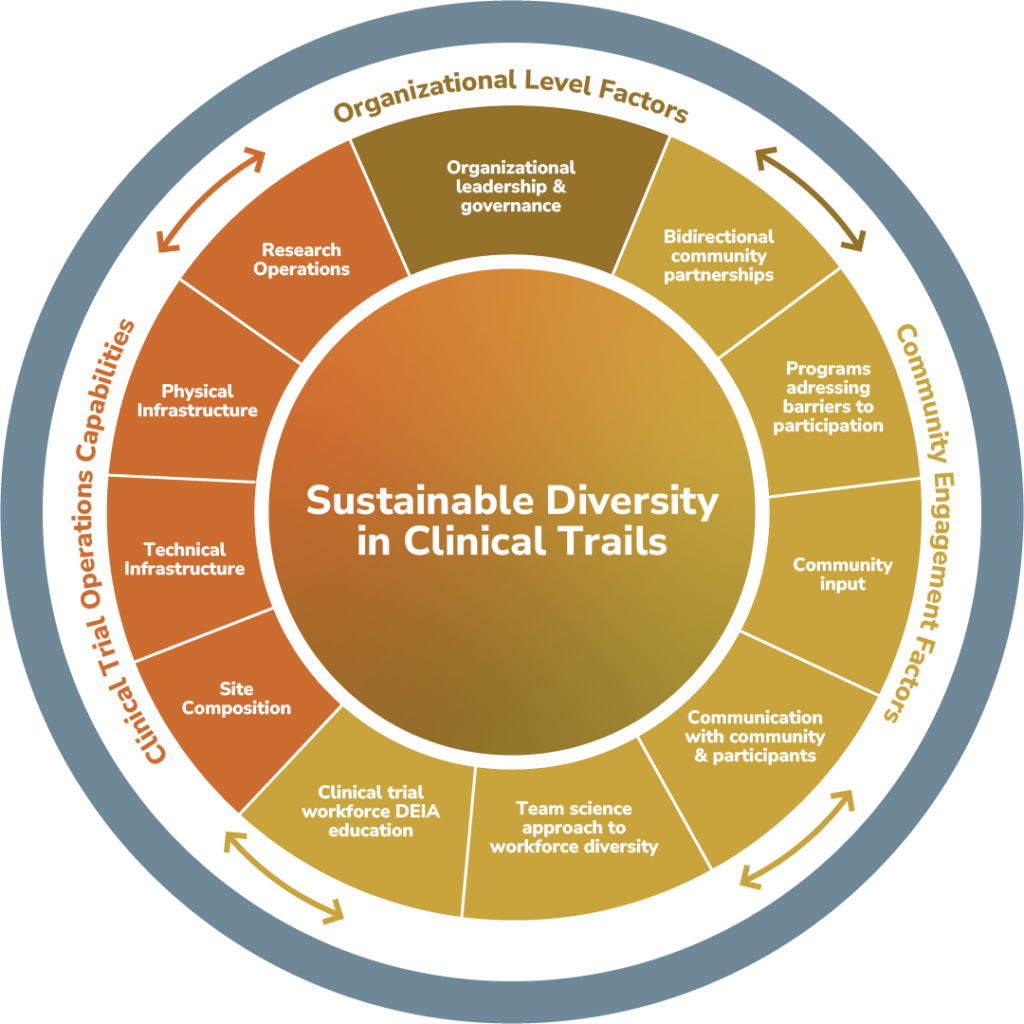
The EQBMED Site Maturity Assessment Model is a holistic, collaborative, site-driven, and formative assessment carried out with potential sites to catalogue their current capabilities and identify opportunities for growth in conducting industry-sponsored clinical trials and expanding access to those trials. It is not intended to be evaluative in nature, or to be used to compare sites in the EQBMED program or otherwise benchmark against others.
The completed assessment will:
- Inform the site-specific roadmap for capability building during the Learning Phase (with the support of infrastructure partners)
- Serve as a baseline for sites to track progress toward their maturity goals
- Create visibility into site capabilities to help trial sponsors assess interest in placing protocols at the site
Importantly, this model draws from and synthesizes prior initiatives including those led by the Yale Center for Clinical Investigation (YCCI), The Clinical Trials Transformation Initiative (CTTI), The National Academy of Medicine, and Multi-Regional Clinical Trials Centers of Brigham and Women’s Hospital and Harvard (MRCT Center).
Citation:
Harris, T., Nunez-Smith, M., Suttiratana, S.C. et al. Supporting diversity in clinical trials: the equitable breakthroughs in medicine site maturity model. Trials 25, 764 (2024). https://doi.org/10.1186/s13063-024-08594-9